INM-088 for the treatment of glaucoma
INM-088 is a topical eye drop formulation under development for the treatment of glaucoma. The active pharmaceutical ingredient (API) in INM-088 is cannabinol, also known as CBN, a rare cannabinoid showing promise in its potential to provide neuroprotection and to reduce intraocular pressure of the eye.
Cannabinol formulation for glaucoma
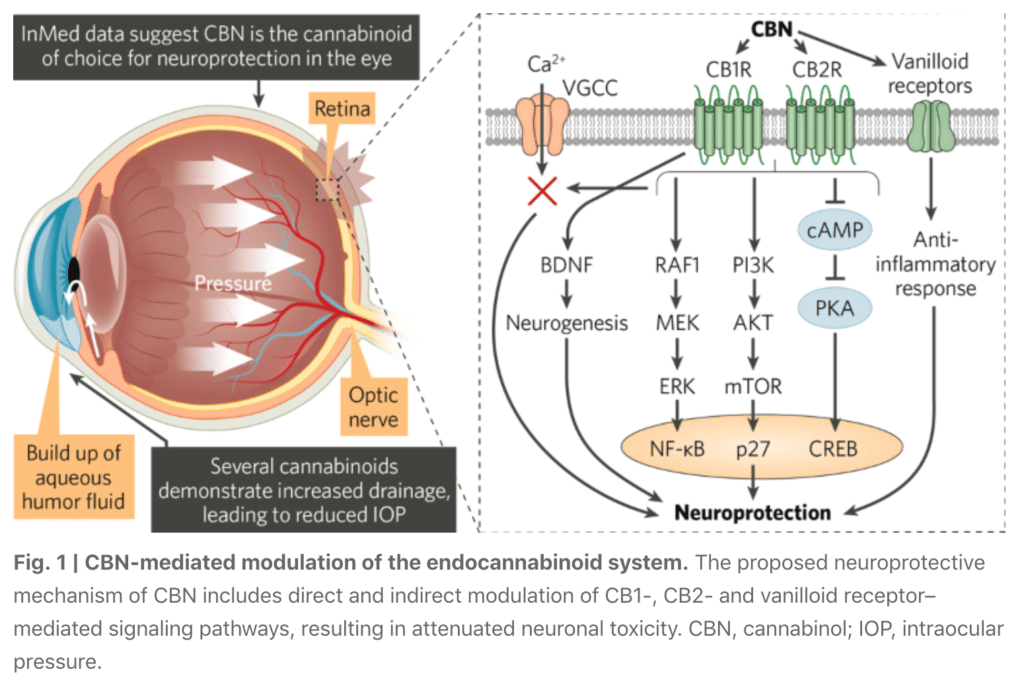
Cannabinol (CBN) is the key active pharmaceutical ingredient (API) in INM-088, which is in preclinical studies as a potential treatment for glaucoma. We are conducting studies to test the ability of CBN to provide protection to the neurons at the back of the eye, referred to as “neuroprotection”, and reduce the intraocular pressure (IOP) in the eye. We compared several cannabinoids, including CBD and THC, to determine which cannabinoid was the best drug candidate for the treatment of glaucoma. Of all of the cannabinoids examined, CBN demonstrated the most optimal effect of neuroprotection. Furthermore, CBN also exhibited intraocular pressure reduction capability.
InMed has completed a pre-Investigational New Drug application discussion with the U.S. Food and Drug Administration regarding manufacturing, preclinical studies and early clinical development plans for INM-088. InMed has gained alignment with the FDA on the design of the initial Phase 1-2 clinical trial to gather preliminary data on the safety and efficacy of INM-088 treatment. Management expects to file regulatory applications in the first half of the calendar year 2024 to initiate a human clinical trial.
INM-088 Study on the Use of CBN as a Potential Treatment for Glaucoma published in Peer-Reviewed Article
InMed’s research of INM-088, a cannabinol formulation being developed for glaucoma, has been published in a peer-reviewed scientific article entitled “Cannabinol Modulates Neuroprotection and Intraocular Pressure: A Potential Multi-Target Therapeutic Intervention for Glaucoma”. The study was published in Biochimica et Biophysical Acta (BBA – Molecular Basis of Disease), a leading international journal focused on biochemistry and molecular genetics of disease processes and models of human disease in the area of aging, cancer, metabolic-, neurological-, and immunological-based diseases.
The peer-reviewed article highlights research evaluating the use of cannabinol, or CBN, as a potential treatment option for glaucoma. Several studies were conducted to evaluate the survival of retinal ganglion cells, modulation of intraocular pressure and its effects on extracellular matrix proteins using in vitro and in vivo glaucoma models.
These studies resulted in two key findings: first, CBN may promote neuroprotection of cells in the retina that are responsible for vision; and second, CBN may normalize intraocular pressure by attenuating changes in the extracellular matrix proteins. The article also reports on the comparison of CBN with other cannabinoids, including cannabidiol (CBD) and tetrahydrocannabinol (THC), with results indicating that CBN has a stronger effect and broader neuroprotective therapeutic range. These observations elucidate the therapeutic potential for CBN in the treatment of glaucoma.
INM-088 key preclinical results as a drug candidate to treat glaucoma
INM-088 is an eye-drop CBN formulation being developed for the treatment of glaucoma. The preclinical development program for INM-088 has included a number of studies comparing a number of cannabinoids, including CBN, THC and CBD, among others, to determine which cannabinoid holds the greatest potential to treat glaucoma. This preclinical research to date is comprised of both in vitro and in vivo studies and led to the selection of CBN as the lead drug candidate for further development.
The scope and results of the in vitro studies to date include the following:
1) Evaluation of the neuroprotective effects of selected cannabinoids on the differentiated retinal ganglion cells, or “RGCs”, a thin layer of neurons responsible for relaying visual signals in the eye, under normal atmospheric pressure and elevated pressure conditions. Elevated pressure mimicking high IOP had a cytotoxic effect on the RGCs. However, the combination of CBN and elevated pressure, within the same time period, resulted in a high level of cell survival in a dose dependent fashion. CBN was superior to both CBD and THC under identical testing conditions. This data indicates a potential neuroprotective effect of CBN in ocular disease.
2) Evaluation of anti-apoptotic effects of CBN on the differentiated RGCs when exposed to elevated pressure conditions. Data indicate that CBN has a significant anti-apoptotic effect on differentiated RGCs subjected to elevated pressure conditions. Exposure of RGC to high pressure for a period of 6 hours led to apoptosis ranging from 30-60%, whereas the addition of CBN resulted in a significantly higher level of cell survival.
3) Evaluation of CBN impact on the expression of specific extracellular matrix (ECM) markers on primary human trabecular meshwork (TM) cells under normal atmospheric pressure, elevated pressure and following stress-induction with Transforming Growth Factor Beta 2 (TGF-ß2), a cytokine used to alter extracellular matrix metabolism. TM cells exposed to CBN under a range of conditions, including normal pressure or elevated pressure or TGF-β2 exposure, for a duration of 72 hours, demonstrated deceased expression of protein markers associated with reduced TM outflow. These results suggest that CBN may have potential to reduce IOP through improvement in the aqueous humor outflow in the TM.
We also conducted several in vivo experiments to understand the pharmacokinetics and efficacy of CBN in the eye as a potential treatment for glaucoma. The scope of these in vivo studies to date include the following:
4) Evaluation of CBN pharmacokinetic profile in the eye and plasma of a preclinical model by direct intravitreal (IVT) injection into the eye. Following IVT delivery, systemic plasma levels of CBN were found to be below detectable limits, whereas CBN levels in the eye were shown to persist for an extended period of time with a projected half-life (t1/2) of approximately 33 hrs. These results present the prospect of high CBN localization at the site of intended treatment.
5) Evaluation of CBN neuroprotective and IOP-lowering effects in a preclinical glaucoma model by IVT injection. Biological function of the neurons was assessed through pattern electroretinogram (“pERG”) measurement of neuronal electrical activity in response to light. Results demonstrated reduced IOP and improvement of pERG function when CBN was delivered by IVT injection after episcleral laser photocoagulation.
Of all the cannabinoids examined in preclinical studies, CBN demonstrated the most optimal neuroprotective effect. Furthermore, CBN also exhibited intraocular pressure reduction capability.
Current treatments for glaucoma primarily focus on decreasing fluid build-up in the eye. Our data has shown that INM-088 may provide neuroprotection in addition to modulating intraocular pressure by improving drainage of fluid in the eye.
InMed is pursuing a formulation for efficient CBN delivery into the eye
InMed has secured an exclusive, worldwide license from EyeCRO LLC for its Microemulsion Drug Ocular Penetration System (“MiDROPS®”) eyedrop delivery technology targeting effective, topical administration of cannabinoids to the eye. InMed has already completed a preliminary investigation demonstrating simplicity, superiority and effectiveness of MiDROPS® to deliver sustained levels of CBN to the eye, as compared to other formulations.
Key design criteria for the INM-088 formulation include, among others:
- Biocompatibility and biodegradability of the formulation;
- Viscous fluid behavior while inside the container (to facilitate ease of manufacturing, handling and dosing);
- Characterized and defined drug release, absorption and subsequent carrier degradation;
- Optimized particle size and surface charge to avoid irritation upon application to the eye and to facilitate ocular penetration; and
- Stable final drug product to ensure drug product quality storage over time.
Ophthalmologists typically prescribe drugs individually and then switch to different classes of drugs on a regular basis in order to prevent the habituation phenomenon (reduction in effect of the drug over time) and negative side effects. There is an opportunity for new therapies with low systemic toxicity and those which may not exhibit habituation.
Designing an ideal delivery system for any ocular disease depends on molecular properties of the drug substance and incorporating it into the formulation while taking into consideration parameters such as size, charge, and affinity towards various ocular tissues and pigments.

Studies show excellent safety profile for cannabinol
As part of our dermatological programs, InMed has completed extensive safety studies of cannabinol, the active pharmaceutical ingredient (API) in INM-088.
InMed’s Phase 1 and 2 clinical trials of INM-755 demonstrated that CBN is safe and well-tolerated. In the Phase 2 trial, there were no serious drug-related adverse events (“AEs”) and there were no withdrawals from treatment. Moderate headaches in one study participant were the only systemic AEs deemed ‘possibly related’ to study drug. Very few local AEs were reported in the treatment areas; they were transient and resolved without cessation of treatment. The Phase 2 trial indicated that INM-755 CBN cream was very well tolerated on sensitive EB skin.
Results from two Phase 1 clinical studies of INM-755 cream in healthy volunteers treated for 14 days indicated that INM-755 cream was safe and well-tolerated on intact skin as well as open epidermal wounds, caused no systemic or serious adverse effects, and there were no subject withdrawals due to adverse events.
Our preclinical studies of cannabinol included doses at levels much higher than what would be used in an ophthalmic pharmaceutical treatment and still demonstrated an excellent safety profile. No drug-related toxicity was demonstrated in any of the studies. Additionally, as INM-088 is being designed for topical delivery to the eye, it would have a localized effect with very little drug being absorbed or migrating into the bloodstream, thus minimizing potential systemic adverse side effects.
Cannabis to treat glaucoma
Decades of anecdotal evidence suggests that the use of Cannabis may play a role in lowering intraocular pressure in glaucoma. However, no such products have been formally investigated in clinical trials and none is currently approved for the treatment of this disease. The neuroprotective role of cannabinoids has not before been utilized as a therapeutic strategy in glaucoma, primarily due to great difficulties associated with the targeted delivery of cannabinoids to intraocular tissues. This class of compound is also relatively poorly bioavailable due to its low aqueous solubility.
Previously reported attempts for topical delivery of cannabinoids, in particular, the psychoactive drug THC, to the ocular tissues used formulations based on mineral oil. Until very recently, studies on novel topical ophthalmic formulations of cannabinoids have been largely non-existent. Nevertheless, the use of marijuana to treat glaucoma has extensive anecdotal evidence and some supporting clinical data. It has been definitively demonstrated and widely appreciated, that smoking marijuana lowers intraocular pressure in both normal individuals and in those with glaucoma. Certain drawbacks are associated with the use of (smoked) marijuana to treat glaucoma:
- Marijuana has a short duration of action (only 3-4 hours), meaning that to lower the intraocular pressure around the clock it would have to be smoked every three hours;
- Marijuana’s mood-altering effects, almost exclusively via the chemical THC, would prevent the patient who is using it from driving, operating heavy machinery, and functioning at maximum mental capacity; and
- Marijuana cigarettes also contain hundreds of compounds that damage the lungs, and the deleterious effect of chronic, frequent use of marijuana upon the brain is well established.
An exciting finding is the discovery of receptors for cannabinoids in the tissues of the eye itself, suggesting that local administration has the possibility of being effective. Furthermore, there is evidence from research in the brain that there may be properties of the cannabinoids that protect nerve cells like those in the optic nerve. This raises the hope that cannabinoids could protect the optic nerve not only through intraocular pressure-lowering but also through a neuroprotective mechanism. However, unless a well-tolerated formulation of a marijuana-related compound with a much longer duration of action is demonstrated in rigorous clinical testing to reduce optic nerve damage and preserve vision, there is no scientific basis for use of these agents in the treatment of glaucoma.
Learn more about the role of cannabinoids in the treatment of glaucoma.
What causes glaucoma?
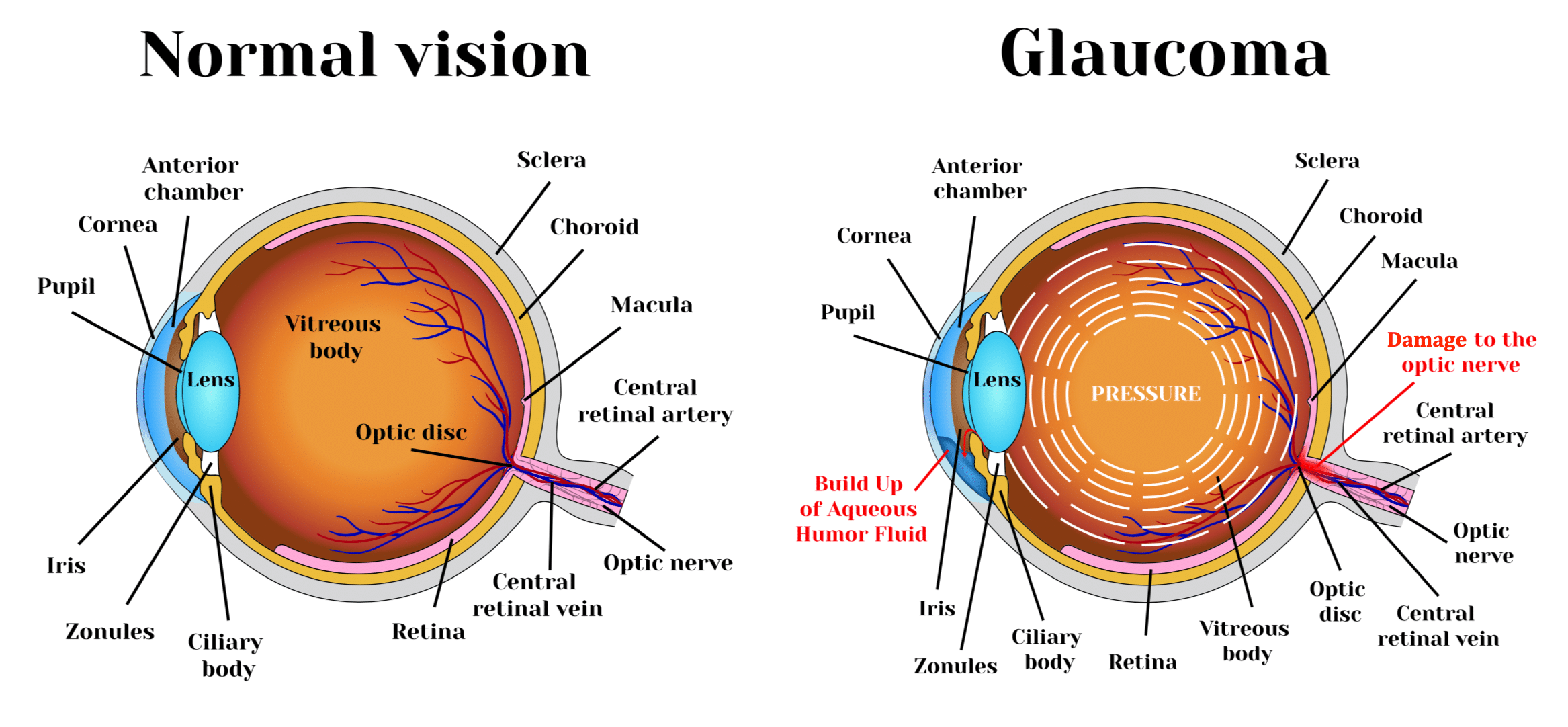
Glaucoma is a chronic optic neuropathy that is typically characterized by high intraocular pressure (IOP). The cause of glaucoma is understood to be inadequate or obstructed drainage of fluid in the eye, or “aqueous humor”, through a drainage membrane called the trabecular meshwork, or “TM”, increasing the fluid pressure within the front part of the eye, or “anterior chamber”, and subsequently leading to pressure at the back part of the eye or “posterior chamber”. The increased intraocular pressure exacts a toll on the nerve cells, called neurons, located at the back of the eye in the retina, thinning the mesh-like tissue in this region and resulting in damage to the neurons and specifically to the optic nerve, which provides the impulses of sight to the brain. This damage leads to blindness. Glaucoma is currently the second leading cause of blindness worldwide and is estimated to affect a population of about 76 million worldwide.
While glaucoma can affect anyone, certain people are at higher risk. According to “Facts about Glaucoma” from the United States National Eye Institute, increased risk factors for glaucoma include individuals with diabetes, hypertension, previous eye injury or a family history of the condition. Individuals at a higher risk also include African American age 40 and older and anyone 60 years of age and older (especially Hispanics/Latinos).
Learn more about the glaucoma prevalence, causes of glaucoma and current treatment methods.
Current treatments for glaucoma
Current treatments for glaucoma include medication, laser treatment and surgery. The goals of glaucoma management are to avoid glaucomatous damage, nerve damage and preserve visual field and total quality of life for patients, with minimal side effects. This requires appropriate diagnostic techniques and follow-up examinations, and judicious selection of treatments for the individual patient. Although intraocular pressure is only one of the major risk factors for glaucoma, lowering it via various pharmaceuticals and/or surgical techniques is currently the mainstay of glaucoma treatment.
Often patients need to take combination of different drugs and multiple eye drops throughout the day. Given side effect profiles, many patients do not take their medications properly or at all. Surgery and laser therapies are intended to physically improve the drainage of fluid from the eyes and lowering of the intraocular pressure. Patients with OAG can have clogged channels in the TM opened with laser therapy, filtering surgery (trabeculectomy) or electrocautery. In other cases, small drainage tubes may be implanted in the eye. Possible complications include pain, redness, infection, inflammation, bleeding, abnormally high or low eye pressure and loss of vision. Some types of eye surgery may accelerate the development of cataracts. Additional procedures may be needed if eye pressure continues to increase.
We believe that there is considerable room for improvement of existing drugs, most of which are formulated as eye drops, in terms of increasing the amount of drug that can be safely delivered to increase its effect, improving the delivery of the drug into the eye, and reducing the common effect in currently used therapies that, over time, their efficacy diminishes as the body becomes tolerant to these classes of drugs.
Studies have shown that when drugs are delivered as eye drops, less than 5% of the dose penetrates into the eye, indicating that 95% of the administered drug never reaches its desired target as it is wiped away upon blinking. Thus, there is much room for improvement on the drug delivery as a means of increasing clinical efficacy.







