IntegraSyn™ - Manufacturing System for Pharmaceutical-Grade Cannabinoids
IntegraSyn™ is InMed’s flexible, integrated cannabinoid manufacturing system
to efficiently produce pharmaceutical-grade, bio-identical cannabinoids.
IntegraSyn™ – An optimized system to cannabinoid manufacturing
IntegraSyn™ is InMed’s integrated cannabinoid synthesis system to manufacture pharmaceutical-grade cannabinoids. Our goal has always been to achieve an efficient, scalable, flexible and economical solution to produce cannabinoids with bio-identical structures to those found in nature. IntegraSyn™ is designed to produce rare cannabinoids for pharmaceutical use. In addition to IntegraSyn™, InMed’s subsidiary, BayMedica, utilizes biosynthesis and chemical synthesis to manufacture rare cannabinoids for the health and wellness industry.
A cost-effective solution to multi-cannabinoid production
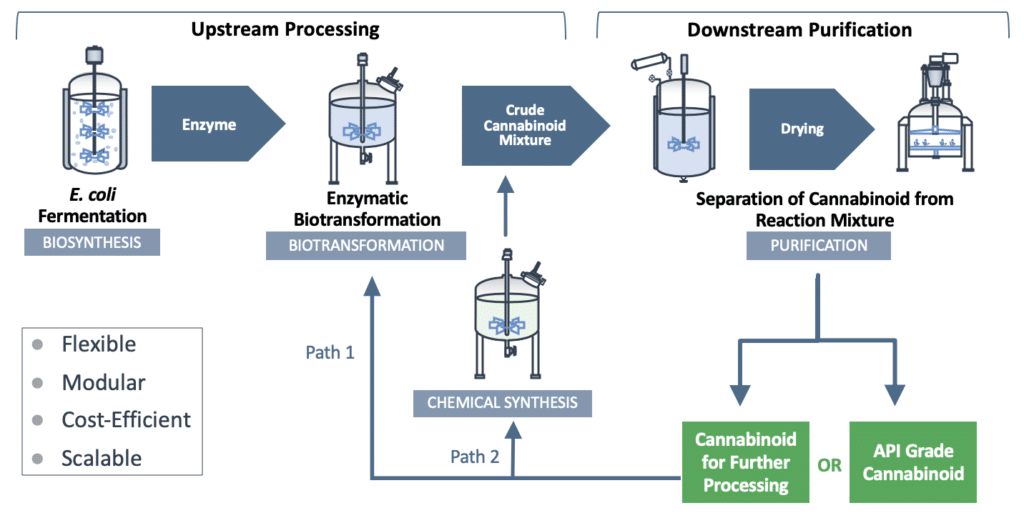
IntegraSyn™ makes cost-efficient use of sophisticated starting materials, requires fewer costly steps all the way through to the end-product, and is designed as a high-yield manufacturing process. We produce high-efficiency enzymes utilizing E. coli biofermentation for use in bulk cannabinoid manufacturing via a biotransformation process and further purification steps. The resulting cannabinoid can be used either as a finished cannabinoid product or as a starting material to produce other cannabinoids using any one of several well-established manufacturing approaches.
Flexibility to shift production of one cannabinoid to a range of cannabinoids
The flexible IntegraSyn™ manufacturing system integrates various pharmaceutical manufacturing processes to optimize production of cannabinoids. While traditional biosynthesis and chemical synthesis focus on production of one cannabinoid from start to finish, the modular IntegraSyn™ process provides flexibility to shift production of one cannabinoid to a range of cannabinoids. This modular approach places less burden on the fermentation microbe and allows for the use of different enzymes and starting materials, leading to optimized yield, time and cost.
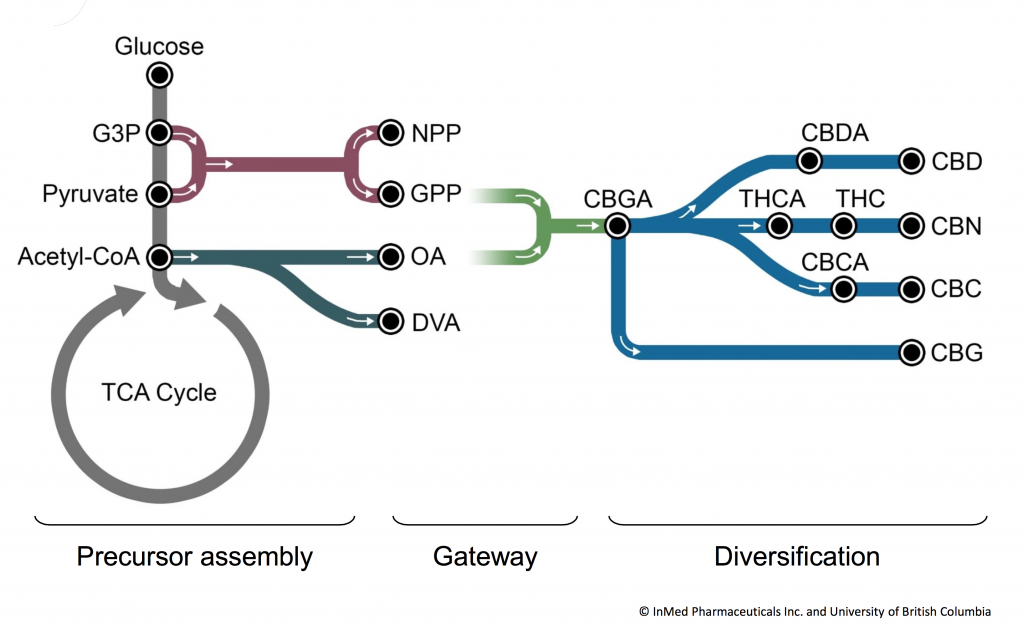
Meeting the cannabinoid needs of our therapeutic programs and future markets
InMed is focused on the development of cannabinoid-based therapeutics to treat serious diseases with high unmet medical needs. We currently have four development programs in dermatological, neurodegenerative and ocular diseases, including INM-755 cannabinol (CBN) cream under development for epidermolysis bullosa, INM-901 under development for the treatment of Alzheimer’s disease, INM-088 under development for glaucoma and INM-089 under development for age-related macular degeneration. IntegraSyn™ has been developed by InMed to supply rare, pharmaceutical-grade, bio-identical cannabinoids for its own development programs and eventual commercial needs.
Gaining access to rare cannabinoids
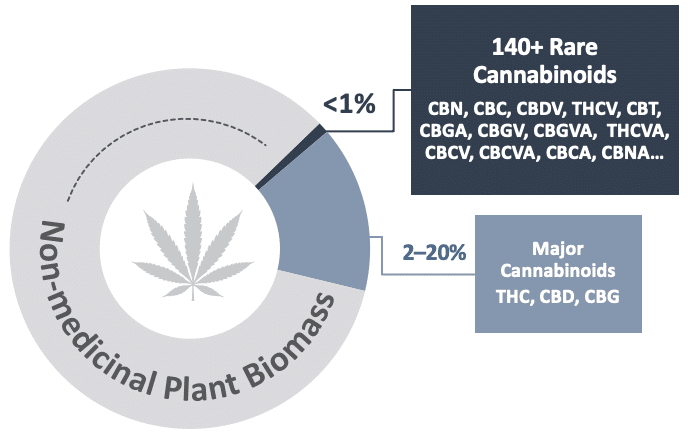
There are more than 100 rare cannabinoids found in only trace amounts in the Cannabis plant – together making up less than 1% of the plant biomass. Evidence suggests that even slight differences in the chemical structure of cannabinoids can result in meaningful differences in biological activity, safety and therapeutic effects in the human body. While rare cannabinoids may hold promise as new treatment options, their low levels in the plant make them impractical to extract for pharmaceutical or any large-scale purpose. IntegraSyn™ was developed to enable access to these rare cannabinoids cost-effectively, at pharmaceutical-grade while being bio-identical to those found in the plant.
Scalable, pharmaceutical-grade cannabinoids to meet future market demand
IntegraSyn™ is designed to be scalable and to produce the consistent, compliant, highly pure cannabinoids required for pharmaceutical products. While having the potential to supply InMed’s own development and future commercialization needs, IntegraSyn™ may be able to supply rare cannabinoids, not previously accessible, to other companies, potentially opening up new product and market opportunities.
Integrated cannabinoid synthesis suitable for long-term needs
Challenges of plant-based Cannabis production, including the large footprint of greenhouses or exterior fields, use of pesticides and fertilizers, security requirements and inconsistent levels of the active compounds, make the synthetic approach of cannabinoid manufacturing a better longer-term strategy for pharmaceutical development and supply. The current traditional method to obtain cannabinoids – plant, grow, harvest, extract and purify – is not feasible for long-term pharmaceutical demand.
A synthetic process provides for a reliable, consistent, scalable and compliant process versus the variability and complexity associated with the extraction and purification of the cannabinoids directly from the plant.
As interest in the therapeutic potential of cannabinoids continues to grow, IntegraSyn™ may be the solution to provide access to a range of cost-effective, bio-identical cannabinoids.