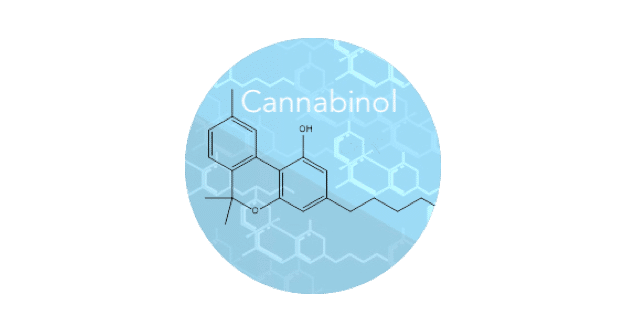
Cannabinol, or CBN for short, is one of the more than 100 rare cannabinoids found in the Cannabis plant. CBN is found in aged Cannabis and is a degradant of tetrahydrocannabinol (THC) after exposure to air, light and heat. Unlike THC, CBN is generally accepted as non-psychoactive.
Cannabinol chemistry
CBN is a stable, highly lipophilic cannabinoid compound. It is insoluble in water, but soluble in organic solvents.
Molecular Formula: C21H26O2
Chemical Names: Cannabinol (CBN), NSC # 134455, Cannabinolo [DCIT], Cannabinolum [INN-Latin]
Chemical Abstract Service number (CAS): 521-35-7
IUPAC Name: 6,6,9-trimethyl-3-pentyl-benzo[c]chromen-1-ol
Molecular Weight: 310.43 g/mol
Boiling Point.: 185°C (365°F) at 0.05 mmHg
Melting Point: 77°C (171°F)
Solubility: Insoluble in water, soluble in methanol, ethanol and other organic solvents
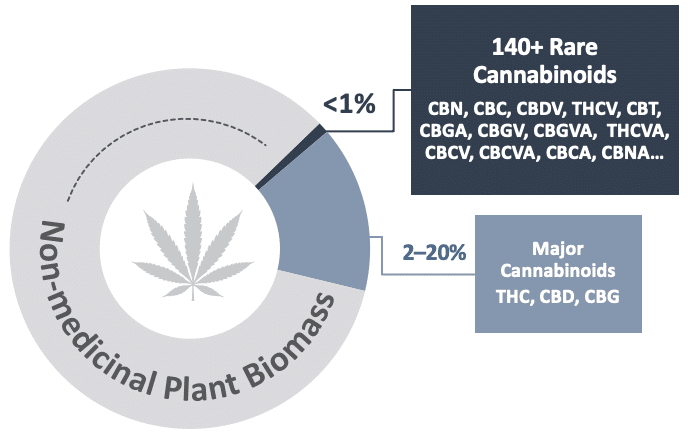
How does cannabinol (CBN) form?

Unlike more prevalent cannabinoids such as THC and cannabidiol (CBD), CBN is not produced by the plant in significant quantities. CBN is mainly formed from the degradation of THC and other cannabinoids, when they are exposed to heat, UV light and/or oxygen. This is the reason why higher levels of CBN can be found in aged, dry Cannabis. The graphic below shows the chemical structure and production of CBN and other cannabinoids in the plant.
Production of CBN and other cannabinoids in the plant
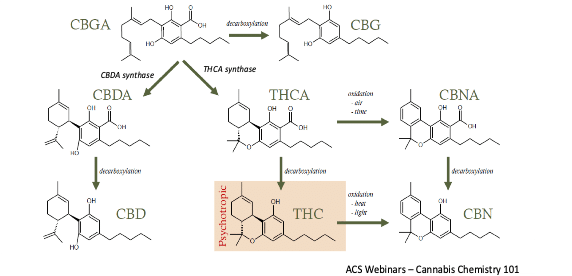
Cannabinol interaction with cannabinoid receptors
The human endocannabinoid system includes several different cannabinoid receptors which regulate many important physiological functions such as immune function, sleep, memory and pain perception. Cannabinoids bind to these receptors and other receptors in the body, signaling the endocannabinoid system into action. The involvement of the endocannabinoid system on many important functions in the body has made cannabinoids a promising target to potentially treat diseases with unmet medical needs.
The two most well-known cannabinoid receptors are the endocannabinoid receptor 1 (CB1), which is more relevant to the central nervous system, and endocannabinoid receptor 2 (CB2), which is associated with immune function. Scientific literature suggests that CBN has low affinity for CB1 receptors and therefore a weak effect on the central nervous system and that CBN preferentially binds to CB2 receptors. Information into the effects of CBN on the endocannabinoid system are limited and InMed continues its research of CBN and how it interacts with the body.
Research into cannabinol’s psychoactive effect
While THC is known to have psychoactive effects, CBN is generally acknowledged as non-psychoactive. However, research to date into the psychoactivity of cannabinoids has been largely focused on the more abundant cannabinoids, THC and CBD, that are inhaled or ingested. Understanding any potential psychoactive effects of CBN has been difficult to achieve by inhalation of Cannabis. With only miniscule amounts of CBN found in the plant, there has been only limited exposure of CBN in humans through marijuana smoking.
InMed’s preclinical studies on central nervous system (CNS) effects
InMed’s formulations are applied topically. This limits the amount of drug that can reach the bloodstream, while still achieving effects at the treatment site.
InMed has conducted several preclinical studies of CBN to test central nervous system effects, as part of the standard safety toxicology package required for drug development. In preclinical studies with subcutaneous administration (under the skin) reaching systemic (blood) levels 10,000 times what is anticipated in topical dosing, no adverse events were seen on central nervous system (CNS) function in a rigorous and extensive evaluation of 108 aspects of behavior posture, gait, and movement.
Potential therapeutic properties of cannabinol
Research into cannabinol’s biological activity has shown a number of therapeutic qualities that may be valuable in developing future medicines for diseases with high unmet need. These studies have indicated a number of promising physiological properties of CBN that include anti-bacterial, anti-inflammatory, neuroprotection, pain relief, appetite stimulation and anti-convulsion.
InMed has conducted a number of preclinical studies demonstrating therapeutic potential in dermatological and ocular diseases. In a preclinical study as part of our lead program, CBN demonstrated activity in reducing markers of inflammation and pain. It also upregulated expression of a type of keratin (keratin 15, or K15), which might lead to skin strengthening and reduced blister formation in epidermolysis bullosa simplex (EBS) patients with mutations of another keratin (keratin 14, or K14). Its anti-inflammatory activity may be beneficial in healing chronic wounds caused by prolonged inflammation. In preclinical studies of InMed’s second program in glaucoma, studies demonstrated the potential of CBN to provide neuroprotection and to reduce intraocular pressure of the eye.
History of cannabinol
Oldest evidence of Cannabis smoking dates back about 2,700 years ago. The discovery was made from the excavation of an ancient tomb in Northern China. Phytochemical analysis of vegetative manner found in a leather basket and bowl was identified as Cannabis Sativa L. and revealed traces of cannabinol.
According to 2006 scientific article entitled “Cannabinoid pharmacology: the first 66 years”, CBN was isolated in the late 1800s, making it the first plant cannabinoid to be isolated. It was then successfully chemically synthesized in 1940.
InMed is the first company to advance cannabinol into a clinical trial as a potential therapeutic to treat disease.