Cannabinoids in Development
We are researching the therapeutic benefits of several rare cannabinoids and have
produced a growing library of rare cannabinoid analogs for development.
Pharmaceutical Development of Rare Cannabinoids
InMed is developing a pipeline of rare cannabinoids across a spectrum of therapeutic applications with large unmet medical needs. Our pharmaceutical pipeline includes programs in dermatology, ocular and Alzheimer’s disease.
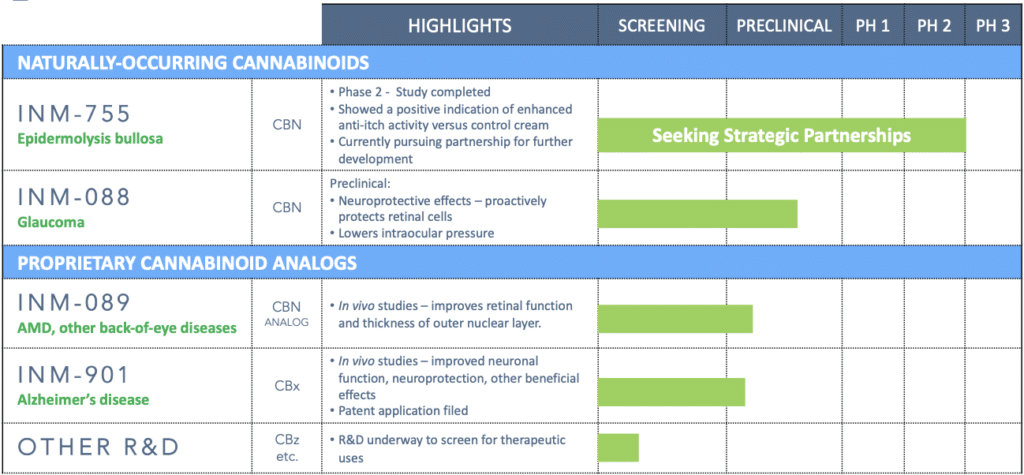
InMed is a leader in the therapeutic development of cannabinol (CBN)
InMed is the first company to advance CBN as a therapeutic product into a clinical trial. CBN is the active pharmaceutical ingredient in two of our pharmaceutical development programs – INM-755 cannabinol cream, which has completed a Phase 2 clinical trial for epidermolysis bullosa, and INM-088 CBN eye drops in preclinical development for glaucoma.
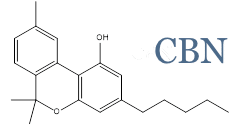
CBN is one of several rare cannabinoids found in the Cannabis plant at significantly lower levels relative to the more common THC and CBD. InMed is exploring the unique physiological effects of CBN, as well as other rare cannabinoids, and their therapeutic potential to treat disease. Results from InMed’s preclinical and clinical studies of CBN have demonstrated a favorable safety and tolerability profile. An exploratory clinical evaluation of the Phase 2 clinical trial data showed a positive indication of enhanced anti-itch activity for INM-755 cannabinol cream versus the control cream alone.
A growing library of new cannabinoid analogs
InMed has an extensive library of cannabinoid new chemical entities for pharmaceutical development, aimed at targeting diverse clinical indications. Years of cannabinoid research and know-how has led to a myriad of patentable cannabinoid analogs. Our analog patents offer broad protection through its unique molecular components, such as side-chain structures. These cannabinoid analogs are suitable for pharmaceutical development.

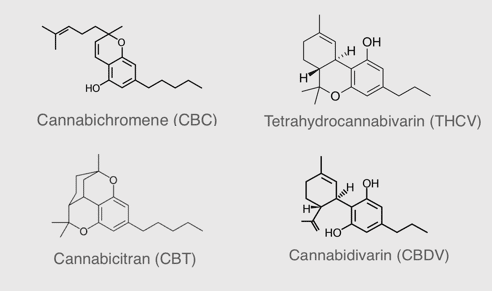
Rare cannabinoids as raw ingredients for the health and wellness industry
InMed’s subsidiary, BayMedica, is manufacturing premium rare cannabinoids for the health and wellness industry. BayMedica is the leading large batch supplier of cannabichromene (CBC) and also has tetrahydrocannabivarin (d9-THCV), cannabidivarin (CBDV) and ultra-rare cannabinoid, cannabicitran (CBTC) for B2B sales to the health and wellness industry. Learn more about our premium quality rare cannabinoid products.
A Diverse Patent Portfolio
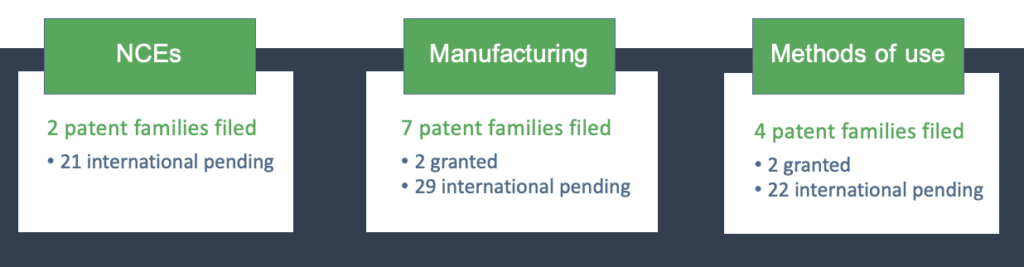
InMed has a strong patent position that includes 13 patent families covering manufacturing, methods of use and formulation of rare cannabinoids. Our portfolio includes a broad patent application for the creation of several variations of novel cannabinoid compounds, biosynthetic pathway and semi-synthetic production of both natural cannabinoids and analogs, methods for manufacturing and uses of specific rare cannabinoids.

Research partnerships that strengthen our cannabinoid knowledge
Our collaborations with cannabinoid experts from around the world ensure we stay at the forefront of rare cannabinoid research and development. Our partners play a key role in the development of our programs and share in our pursuit of understanding the biological activity and therapeutic relevance of rare cannabinoids and demonstrating the preclinical and clinical evidence that will allow for its safe use.
The therapeutic potential of rare cannabinoids
Each cannabinoid has a specific chemical structure that confers unique physiological properties in humans. Evidence demonstrates that even slight differences in the structure of these compounds can result in profound differences in biological activity, safety and potential therapeutic effect in the human body.
InMed is researching and developing rare cannabinoids as potential therapeutics for unmet medical needs. While the most prevalent cannabinoids, tetrahydrocannabinol (THC) and cannabidiol (CBD), are widely used for a variety of reasons, evidence suggests there may be greater therapeutic potential in rare cannabinoids that are found in very small traces in the Cannabis plant.