How do cannabinoids interact with the human body?
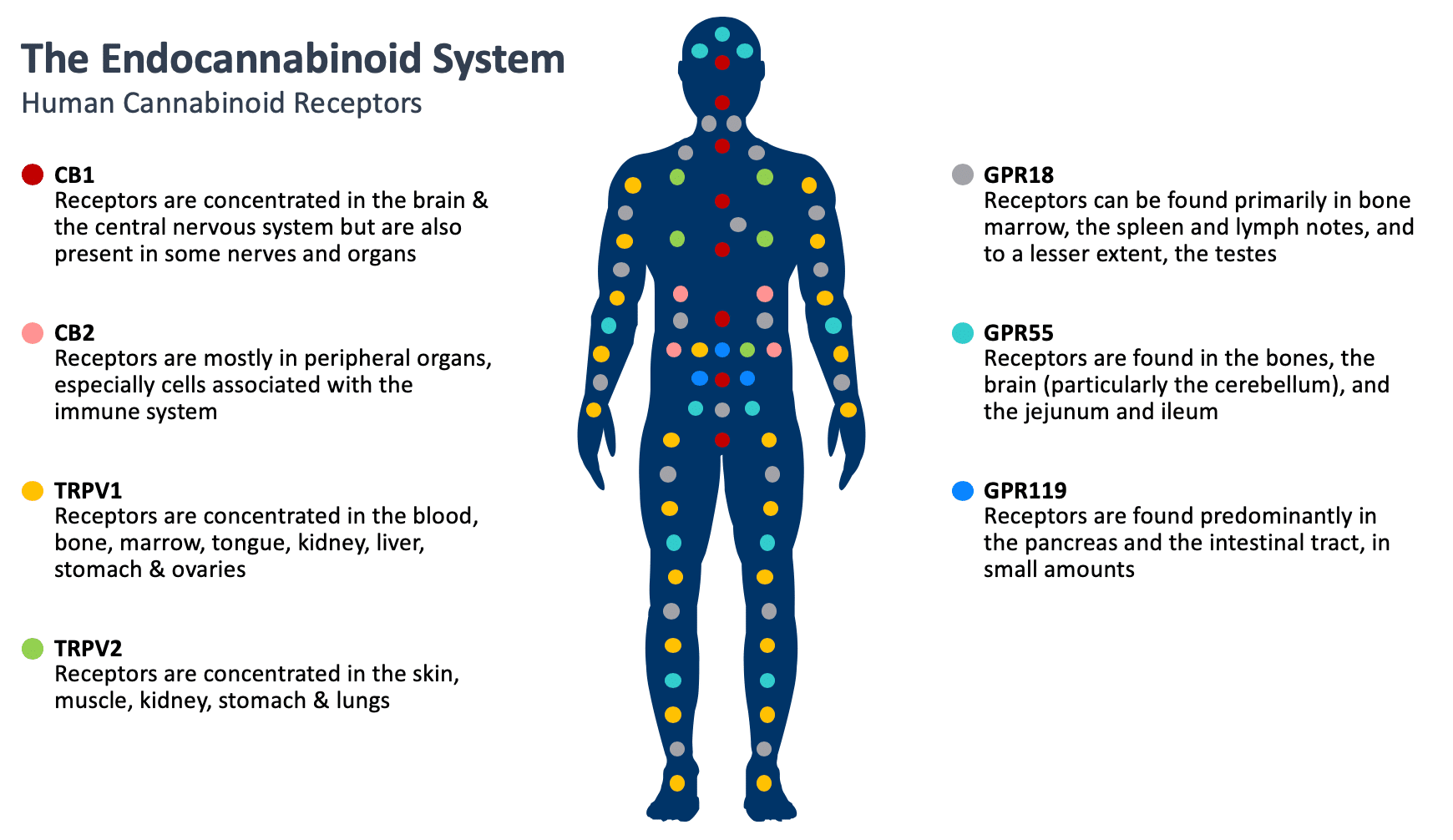
There has been a significant growth in interest and research into the potential therapeutic properties of cannabinoids. It starts with how cannabinoids act on cannabinoid receptors, which are found throughout the human body and are involved in many physiological functions such as pain perception, memory, appetite and immune function. Cannabinoids can bind to cannabinoid receptors, as well as other receptors, signaling the endocannabinoid system into action. The relevance of the endocannabinoid system on many important physiological processes has made cannabinoids an important target to potentially treat a number of diseases and symptoms.
Cannabinol is a rare cannabinoid showing therapeutic potential
Many people are familiar with the two most common cannabinoids – tetrahydrocannabinol (THC) and cannabidiol (CBD). THC and CBD are the most prevalent cannabinoids in the Cannabis plant. These two cannabinoids are traditionally extracted from the plant and have been used for many different purposes from recreational and medicinal use to food and beauty products.
While interest in the potential uses of cannabinoids continues to expand, there is a growing body of research into the potential therapeutic benefits of other cannabinoids, beyond THC and CBD. There are more than 100 rare cannabinoids in the plant. In total, these rare cannabinoids make up less than 1% of the plant. Each cannabinoid, even if only slightly different in molecular structure from each other, can have distinctly different therapeutic qualities. One of those rare cannabinoids showing therapeutic promise is cannabinol or CBN for short.
What is known about CBN?
CBN is found in aged Cannabis and is a degradant of tetrahydrocannabinol after exposure to air, light and/or heat. However, unlike THC which has known psychoactive effects, research so far has shown that CBN is generally accepted as non-psychoactive. This makes CBN an attractive target for therapeutic research.
Investigations into the potential effects of rare cannabinoids are very limited. We’ve only just started to see increased research into the potential therapeutic uses of rare cannabinoids, such as CBN. Much of the historical research has been based on anecdotal or observational studies. Most recently, InMed Pharmaceuticals has conducted several preclinical safety and efficacy studies of CBN and has now completed two Phase 1 safety studies in healthy volunteers, making it the first clinical trial of cannabinol in humans.
Cannabinol interaction with cannabinoid receptors
While there are numerous cannabinoid receptors in the human body, the two most well-known are the cannabinoid receptor 1 (CB1), which is more significant to the central nervous system, and cannabinoid receptor 2 (CB2), which is more common with the immune system.
Research to date suggests that CBN has low affinity for CB1 receptors and therefore a weak effect on the central nervous system and that CBN preferentially binds to CB2 receptors. Information into the effects of CBN on the endocannabinoid system are limited and InMed continues its research of CBN and how it interacts with the body.
What is CBN good for? How is CBN used?
In preclinical models, CBN has been reported to show a number of properties with therapeutic potential such as anti-bacterial, anti-inflammatory, neuroprotection, pain relief, appetite stimulation and anti-convulsion effects.
What studies of CBN have been conducted?
CBN as an anti-bacterial agent
In a research article published in Nature in 2008, five cannabinoids including cannabinol, cannabigerol, THC, CBD and cannabichromene showed potent anti-bacterial activity. These five cannabinoids were tested against a variety of methicillin-resistant Staphylococcus aureus (MRSA) strains.
Article: Antibacterial cannabinoids from Cannabis sativa: a structure-activity study
CBN has an anti-inflammatory agent
In the article “Cannabinoids as novel anti-inflammatory drugs” published in Future Medicinal Chemistry in October 2009, a study using cannabinol in OVA-sensitized and challenged A/J mice demonstrated decreased allergen-induced mucus production.
In preclinical pharmacology studies conducted by InMed, CBN demonstrated activity in reducing markers of inflammation.
Article: Cannabinoids as novel anti-inflammatory drugs
CBN for neuroprotection
In a published article in the Journal of Pharmacology Experimental Therapeutics in June 2000, a number of cannabinoids including cannabinol were shown to prevent serum-deprived cell death. The study suggests that these cannabinoids act as antioxidants to modulate cell survival.
InMed has conducted a number of preclinical studies in its glaucoma program comparing CBN with several cannabinoids, including CBD and THC, and CBN demonstrated the most optimal neuroprotective effect.
Article: Cannabinoids protect cells from oxidative cell death: a receptor-independent mechanism
CBN for pain relief
Preclinical studies suggest that cannabinol may have pain relieving properties. An article published in the Journal of Neuroscience in June 2002 indicates that cannabinol activates capsaicin-sensitive sensory nerves. Cannabinol causes vasorelaxation (reduction of vascular tension), which resolves in arteries pretreated with capsaicin. In the study, cannabinol was shown to release CGRP from capsaicin-sensitive sensory nerves. CGRP (calcitonin gene-related peptide) is a protein involved in inflammation and pain in the nervous system.
Preclinical studies of conducted by InMed demonstrated that CBN reduced markers of inflammation and pain.
CBN as an appetite stimulant
A 2012 study published in Psychopharmacology assessed the effects of a number of cannabinoids, including cannabinol, on feeding behaviors. In the study, cannabinol impacted feeding behaviors resulting in increased meal size and duration. Cannabinol also significantly increased the intake during hour 1 and total food consumed during the test. Conversely, cannabidiol significantly reduced total chow consumption over the test period. Cannabigerol administration induced no changes to feeding behavior.
Article: Cannabinol and cannabidiol exert opposing effects on rat feeding patterns
CBN as an anti-convulsant
Recent research is looking into the anti-convulsant effects of cannabinoids, which may show promise as potential treatments for epilepsy. A study comparing THC, CBD and CBN looked into the anti-convulsant effects in mice. The study concluded that all three cannabinoids were effective anticonvulsants; THC and CBD had higher potencies, relative to CBN.
Article: The anticonvulsant activity of cannabidiol and cannabinol
CBN as a potential treatment in managing symptoms of epidermolysis bullosa (EB) as well as improving skin integrity in a subset of EB patients
In preclinical pharmacology studies, CBN demonstrated activity in reducing markers of inflammation and pain. It also upregulated expression of a type of keratin (keratin 15, or K15), which might lead to improved skin integrity and reduced blister formation in EB simplex (EBS) patients with mutations of another keratin (keratin 14, or K14). Its anti-inflammatory activity may be beneficial in healing chronic wounds where healing has been prevented by prolonged inflammation.
InMed’s INM-755 cream is the first cannabinol formulation under therapeutic development to advance to clinical trials. InMed has commenced a Phase 2 clinical trial of INM-755 (cannabinol) cream in Epidermolysis Bullosa (“EB”).
InMed has completed two Phase 1 studies of INM-755 cream, including treatment on intact skin and treatment on wounded skin, both in healthy volunteers. Results from two Phase 1 clinical studies of INM-755 cream in healthy volunteers treated for 14 days indicated that INM-755 cream was safe and well-tolerated on intact skin as well as open epidermal wounds, caused no systemic or serious adverse effects, and there were no subject withdrawals due to adverse events.
CBN eye drop formulation being studied for glaucoma
Cannabinol (CBN) is the key active pharmaceutical ingredient (API) in INM-088, which is in preclinical studies as a potential treatment for glaucoma. We are conducting studies to test the ability of CBN to provide protection to the neurons at the back of the eye, referred to as “neuroprotection”, and reduce the intraocular pressure in the eye. We compared several cannabinoids, including CBD and THC, to determine which cannabinoid was the best drug candidate for the treatment of glaucoma. Of all of the cannabinoids examined, CBN demonstrated the most optimal effect of neuroprotection. Furthermore, CBN also exhibited intraocular pressure reduction capability.
Central nervous system (CNS) safety studies on cannabinol
InMed’s current research and clinical formulations are applied topically. This limits the amount of drug that can reach the bloodstream, while still achieving effects at the treatment site.
InMed has conducted several preclinical studies of CBN to test central nervous system effects, as part of the standard safety toxicology package required for drug development. In preclinical studies in which the drug was administered by injection in order to reach blood levels 10,000 times more than what is anticipated in topical dosing, no adverse events were seen on central nervous system (CNS) function. This study was a rigorous and extensive evaluation of 108 aspects of behavior posture, gait, and movement.
Read more about InMed’s safety studies: https://www.inmedpharma.com/science/inmed-studies/
Statements contained in this article have not been evaluated by the U.S. Food and Drug Administration (FDA) and are not intended to diagnose, treat or cure any disease. The studies mentioned in this article have not been conducted under formal human clinical trials or reviewed by a regulatory agency. The results of the studies referenced in this article have not been proven to diagnose or treat any disease. Always check with your healthcare practitioner before starting any new treatment. Readers are cautioned that the research contained in the article is not exhaustive. InMed Pharmaceuticals Inc. undertakes no obligation to update the information contained in the article, except as required by law.